L1-4 suspension.pdf for pharmhacists and
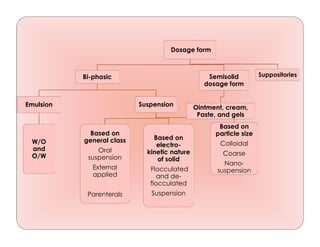
- 1. Dosage form Bi-phasic Emulsion W/O and O/W Suspension Based on general class Oral suspension External applied Parenterals Based on electro- kinetic nature of solid Flocculated and de- flocculated Suspension Based on particle size Colloidal Coarse Nano- suspension Semisolid dosage form Ointment, cream, Paste, and gels Suppositories
- 2. Dr. Shahid Jami, Ph.D. Email: shahid.jamil@knu.edu.iq COLLEGE OF PHARMACY B PHARM III STAGE (VI SEM) PPH651 PHARMACEUTICALTECHNOLOGY II
- 3. SUSPENSIONS PHARMACEUTICAL SUSPENSIONS DR SHAHID JAMIL DEPARTMENT OF PHARMACEUTICS E Mail ID : shahid.jamil@knu.edu.iq
- 4. SUSPENSIONS Definition. Classification. Advantages & disadvantages. Applications. Theoretic consideration of suspensions. •Sedimentation •Brownian movement •Electrokinetic properties CONTENTS
- 5. SUSPENSIONS Flocculated and deflocculated suspension Formulation of suspensions Preparation of suspensions Packing of suspensions Storage requirement & labelling Stability problem of suspension Evaluation of suspension 5
- 7. SUSPENSIONS 7 The term "Disperse System" refers to a system in which one substance (The Dispersed Phase) is distributed, in discrete units, throughout a second substance (the continuous Phase ). Each phase can exist in solid, liquid, or gaseous state . Suspensions are heterogenous system consisting of 2 phases. DISPERSE SYSTEM
- 8. SUSPENSIONS 8 A solid in liquid dispersion in which the particles are of colloidal size. DISPERSE SYSTEM DISPERSED MEDIUM DISPERSED PHASE oAqueous /oily liquid oInsoluble solid
- 9. SUSPENSIONS Definition A Pharmaceutical suspension is a coarse dispersion in which internal phase (therapeutically active ingredient)is dispersed uniformly throughout the external phase. 9
- 10. SUSPENSIONS The internal phase consisting of insoluble solid particles having a range of size(0.5 to 5 microns) which is maintained uniformly through out the suspending vehicle with aid of single or combination of suspending agent. The external phase (suspending medium) is generally aqueous in some instance, may be an organic or oily liquid for non oral use. 10
- 11. SUSPENSIONS Applications Suspension is usually applicable for drug which is insoluble (or ) poorly soluble. E.g. Prednisolone suspension To prevent degradation of drug or to improve stability of drug. E.g. Oxy tetracycline suspension To mask the taste of bitter of unpleasant drug. E.g. Chloramphenicol palmitate suspension Suspension of drug can be formulated for topical application e.g. Calamine lotion 11
- 12. SUSPENSIONS Suspension can be formulated for parentral application in order to control rate of drug absorption. E.g. penicillin procaine Vaccines as a immunizing agent are often formulated as suspension. E.g. Cholera vaccine X-ray contrast agent are also formulated as suspension . eg: Barium sulphate for examination of alimentary tract. 12
- 13. SUSPENSIONS Oral suspension eg: Paracetamol suspension antacids, Tetracycline HCl. Externally applied suspension eg :Calamine lotion. Parenteral suspension eg: Procaine penicillin G Insulin Zinc Suspension Classification Based On General Classes 13
- 14. SUSPENSIONS Based on Proportion of Solid Particles Dilute suspension (2 to10%w/v solid) Eg: cortisone acetate, predinisolone acetate Concentrated suspension (50%w/v solid) Eg: zinc oxide suspension 14
- 15. SUSPENSIONS Based on Electrokinetic Nature of Solid Particles Flocculated suspension Deflocculated suspension 15
- 16. SUSPENSIONS Based on Size of Solid Particles Colloidal suspensions (< 1 micron) -Suspensions having particle sizes of suspended solid less than about 1micron in size are called as colloidal suspensions. 16
- 17. SUSPENSIONS Coarse suspensions (>1 micron) Suspensions having particle sizes of greater than about 1micron in diameter are called as coarse suspensions. Suspensions are the biphasic colloidal dispersions of nanosized drug particles, stabilized by surfactants. Size of the drug particles is less than 1mm. Nano suspensions (10 ng) Coarse dispersion Barium sulphate 17
- 18. SUSPENSIONS Advantages And Disadvantages .Suspension can improve chemical stability of certain drug. E.g. Procaine penicillin G. Drug in suspension exhibits higher rate of bioavailability than other dosage forms. Solution > Suspension > Capsule > Compressed Tablet > Coated tablet Duration and onset of action can be controlled. E.g. Protamine Zinc-Insulin suspension. Suspension can mask the unpleasant/ bitter taste of drug. E.g. Chloramphenicol 18 Advantages
- 19. SUSPENSIONS Physical stability , sedimentation and compaction can causes problems. It is bulky, sufficient care must be taken during handling and transport. It is difficult to formulate. Uniform and accurate dose can not be achieved unless suspension are packed in unit dosage form. 19 Disadvantages
- 20. SUSPENSIONS The suspended particles should not settle rapidly and sediment produced, must be easily re-suspended by the use of moderate amount of shaking. It should be easy to pour yet not watery and no grittiness. It should have pleasing odour , colour and palatability. Good syringeability. It should be physically,chemically and microbiologically stable. Parenteral /Ophthalmic suspension should be sterilizable. Ideal properties of Pharmaceutical Suspensions 20
- 21. SUSPENSIONS Some theoretic considerations are : Particle size control. Wetting Sedimentation Brownian movement Electokinetic Aggregation 21 THEORITICAL CONSIDERATION OF SUSPENSIONS
- 22. SUSPENSIONS 22 Particle size control: - Particle size of any suspension is critical and must be reduced within the range . -Too large or too small particles should be avoided. Larger particles will: settle faster at the bottom of the container particles > 5 um impart a gritty texture to the product and also cause irritation if injected or instilled to the eye particles > 25 um may block the needle -Too fine particles will easily form hard cake at the bottom of the container.
- 23. SUSPENSIONS 23 Wetting of the particles Hydrophilic materials (talc, ZnO, Mg2CO3) are easily wetted by water while hydrophobic materials (sulphur , charcoal) are not due to the layer of adsorbed air on the surface. Thus, the particles, even high density, float on the surface of the liquid until the layer of air is displaced completely. The use of wetting agent allows removing this air from the surface and to easy penetration of the vehicle into the pores. However hydrophobic materials are easily wetted by non-polar liquids.
- 24. SUSPENSIONS THEORY OF SEDIMENTATION SEDIMENTATION: Sedimentation means settling of particle (or) floccules occur under gravitational force in liquid dosage form. 24
- 25. SUSPENSIONS 2.1. Velocity of sedimentation expressed by Stoke’s equation Where, d = Diameter of particle r = radius of particle vsed.= sedimentation velocity in cm / sec ρ s= density of disperse phase ρ o= density of disperse media g = acceleration due to gravity η o = viscosity of disperse medium in poise 25
- 26. SUSPENSIONS Limitation Of Stoke’s Equation . Stoke's equation applies only to: Spherical particles in a very dilute suspension (0.5 to 2 gm per 100 ml) Particles which freely settle without collision . Particles with no physical or chemical attraction. 26
- 27. SUSPENSIONS Sedimentation Parameters 27 Sedimentation volume (F) or height (H) for flocculated suspensions: Definition: Sedimentation volume is a ratio of the ultimate volume of sediment (Vu) to the original volume of sediment (VO) before settling. F = V u / VO Where, Vu = final or ultimate volume of sediment VO = original volume of suspension before settling
- 28. SUSPENSIONS 28 F has values ranging from less than one to greater than one. When F < 1 Vu < Vo When F =1 Vu = Vo The system is in flocculated equilibrium and show no clear supernatant on standing. When F > 1 Vu > Vo Sediment volume is greater than the original volume due to the network of flocs formed in the suspension and so loose and fluffy sediment
- 29. SUSPENSIONS The sedimentation volume gives only a qualitative account of flocculation. Fig : Suspensions quantified by sedimentation volume (f) 29
- 30. SUSPENSIONS 30 Degree of flocculation (β) It is the ratio of the sedimentation volume of the flocculated suspension ,F , to the sedimentation volume of the deflocculated suspension, F∞ ß = F / F∞ (Vu/Vo) flocculated ß = -------------------- (Vu/Vo) deflocculated The minimum value of ß is 1,when flocculated suspension sedimentation volume is equal to the sedimentation volume of deflocculated suspension.
- 31. SUSPENSIONS 31 Example: Compute the sedimentation volume of a 5% w/v suspension of magnesium carbonate in water.The initial volume isVo = 100 mL and the final volume of the sediment isVu = 30 mL. If the degree of flocculation is β =1.3, what is the deflocculated sedimentation volume, F∞? F =Vu /Vo F = 30/100 = 0.3 β = F / F∞ 1.3 = 0.3 / F∞ F∞ = 0.23
- 32. • Work must be done to reduce a solid to small particles and disperse them in a continuous medium. • The large surface area of the particles that results from the comminution is associated with a surface free energy that makes the system thermodynamically unstable. • by which we mean that the particles are highly energetic and tend to regroup in such a way as to decrease the total area and reduce the surface free energy. • An increase in the work, W, or surface free energy, ΔG, brought about by dividing the solid into smaller particles and consequently increasing the total surface area, ΔA, is given by ΔG= γSL. ΔA • where γSL is the interfacial tension between the liquid medium and the solid particles Surface free energy
- 33. . 33 20 Thermodynamic and kinetic stability of dispersed systems Brownian Movement Brownian movement of particle prevents sedimentation by keeping the dispersed material in random motion. Brownian movement depends on the density of dispersed phase and the density and viscosity of the disperse medium. The kinetic bombardment of the particles by the molecules of the suspending medium will keep the particles suspending, provided that their size is below critical radius (r).
- 34. SUSPENSIONS Brownian movement can be observed, If particle size is about 2 to 5mm, When the density of particle & viscosity of medium are favorable. 34
- 35. Electrical Properties of Interfaces 35 20
- 36. Electrical Properties of Interfaces: Electric double layer 2018-4-14 • Consider solid surface in contact with solution of electrolyte containing ions • Some cations (+) adsorb on solid surface • Adsorbe ions that give charge to surface aa' (in this case cations +) known as potential determining ions. • Anions attracted to positive charge by electrical force of attraction known as counter ions or gegenions . • Shear plane is bb' rather than aa' because of tightly bound layer • First layer is aa' to bb' • Second layer is bb' to cc'... more negative chargr is present in this layer in this case.
- 38. Nerst potential 2018-4-14 • Potential at solid surface due to aa' due to potential determining ions is known as nerst potential • It is define as potential difference between actual surface and electro nutral region
- 39. Zeta potential 2018-4-14 Ø The zeta potential is defined as the difference in potential between the surface of the tightly bound layer (shear plane) and electro- neutral region of the solution. Ø Zeta potential has practical application in stability of systems containing dispersed particles .
- 40. 26 26 Ø If the zeta potential is reduced below a certain value, the attractive forces exceed the repulsive forces, and the particles come together. This phenomenon is known as flocculation Ø The flocculated suspension is one in which zeta potential of particle is -20 to +20 mV Ø Thus the phenomenon of flocculation and de-flocculation depends on zeta potential carried by particles. Zeta potential
- 42. SUSPENSIONS 42 Ø The potential at the solid surface aa′ due to the potential-determining ion is theelectrothermodynamic (Nernst) potential, E, and is defined as the difference in potential between the actual surface and theelectroneutral region of the solution. Ø The potential located at the shear plane bb′ is known as the electrokinetic, or zeta, potential, Z. The zeta potential is defined as the difference in potential between the surface of the tightly bound layer (shear plane) and the electroneutral region of the solution. As shown in Figure , the potential initially drops off rapidly, followed by a more gradual decrease as the distance from the surface increases. This is because the counterions close to the surface act as a screen that reduces the electrostatic attraction between the charged surface and those counterions further away from the surface. The zeta potentials are positive (Z1), zero (Z2), and negative (Z3).
- 43. SUSPENSIONS 43 Deflocculation and flocculation Flocculated Suspensions In flocculated suspension, formed flocs (loose aggregates) will cause increase in sedimentation rate due to increase in size of sedimenting particles. Hence, flocculated suspensions sediment more rapidly. Here, the sedimentation depends not only on the size of the flocs but also on the porosity of flocs.
- 44. SUSPENSIONS 44 Deflocculated suspensions In deflocculated suspension, individual particles are settling. Rate of sedimentation is slow , which prevents entrapping of liquid medium which makes it difficult to re-disperse by agitation. This phenomenon called ‘caking’ or ‘claying’. In deflocculated suspension larger particles settle fast and smaller remain in supernatant liquid so supernatant appears cloudy.
- 45. DLVO theory 2018-4-14 • DLVO theory was developed in the 1940s and named after the • Russian scientists – B. Derjaguin – L. Landau, • Dutch scientists – E. Verwey – J. Overbeek),
- 46. DLVO 2018-4-14 • It proposed that an energy barrier resulting from the electrostatic repulsive force prevents two particles approaching one another and adhering together. • If the particles collide with sufficient energy to overcome the barrier, • the Van der Waals attractive force will attract them strongly and cause them adhere together irreversibly. • If the particles repel each other strongly, the dispersion will resist coagulation and the dispersed system will be stable. • If the repulsion is not sufficient then coagulation will take place.
- 49. Need of Controlled Flocculation 2018-4-14 • Assume powder is properly wetted and dispersed • In order to prevent compact sediment we need controlled flocculation
- 50. Controlled Flocculation 2018-4-14 • Electrolytes (ionic substance) act as flocculating agents by reducing electrical barrier between particles... by decresing zeta potential and forming bridge between adjascent particles • Surfactant • Polymer
- 51. • At low electrolyte conc --Repulsive force predominate • At high electrolyte conc --Repulsive force reduce and cause coagulation Effect of electrolytes 2018-4-14
- 53. Effect of electrolytes 2018-4-14 • Bismuth sub nitrate particles posses +ve charge • If we add monobasic potassium phosphate (KH2PO4) then positive zeta potential decrease to zero because of adsorption of -ve phosphate ions then increase in negative direction • At certain +ve zeta potential, maximum flocculation occur
- 54. Effect of electrolytes 2018-4-14 • Onset of flocculation coincide with maximum sedimentation volume • When zeta potential become sufficiently -ve repeptization (deflocculation) occur once again and sedimentation volume(F) falls
- 55. Effect of Surfactant 2018-4-14 • Surfactant improve dispersion by reducing surface tension • Act as wetting and deflocculating agent • Ionic surfactant (SLS) sometime cause flocculation
- 56. Effect of Polymers 2018-4-14 • Act as flocculating agent • Chain of polymer adsorb on multiple particles • Ex. Xanthum gum