Chemistry Core Notes Edexcel.
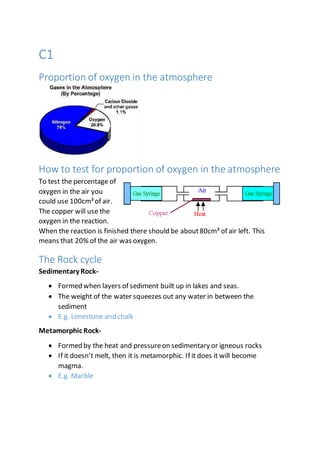
- 1. C1 Proportion of oxygen in the atmosphere How to test for proportion of oxygen in the atmosphere To test the percentage of oxygen in the air you could use 100cm³of air. The copper will use the oxygen in the reaction. When the reaction is finished there should be about80cm³ of air left. This means that 20% of the air was oxygen. The Rock cycle Sedimentary Rock- Formed when layers of sediment built up in lakes and seas. The weight of the water squeezes out any water in between the sediment E.g. Limestone and chalk Metamorphic Rock- Formed by the heat and pressureon sedimentary or igneous rocks If it doesn’t melt, then it is metamorphic. If it does it will become magma. E.g. Marble
- 2. Igneous Rock- Formed when magma cools down If it cools quickly, it is extrusive. This means it will have small crystals If it cools slowly, it is intrusive. This means it will have large crystals E.g. Granite Thermal decomposition How a substancedecomposes when heat is applied. For example: Calcium carbonate calcium oxide + carbon dioxide When Carbonates are thermally decomposed, carbon dioxide is formed. Metal Carbonates formtheir oxide and Carbon dioxide E.g. Zinc Carbonatecan be heated to formZinc oxide and Carbon dioxide Neutralisation Reaction Acid = PH lower than 7 Base = PH higher than 7 Alkali = A basethat is dissolved in water How can Calcium Hydroxide be produced When water is added to calcium oxide, Calcium hydroxideis formed. E.g. Calcium is an Alkali and can be used to quickly neutralise acidic soil. It can also be dissolved in water to formlime water. State Symbols PH lower than 7 PH Higher than 7
- 3. Salt formation Salts are formed through the neutralisation reaction. However, different salts are formed from different acids. An Acid and a Metal makes a salt Metal Carbonates e.g. HazardSymbols Metal Oxides and Metal Hydroxides Metal oxides and Metal hydroxides areoften basses which means it can be neutralised with acids to forma salt and water
- 4. Electrolysis DC currentapplied to an electrolyte. Positively charged ions will be attracted to the cathode (-). Negatively charged ions will be attracted to the anode (+). This is because oppositecharges attract. Chlorine Chlorine can be produced by electrolysing sodiumchloride solution. Chlorine can be used to treat water supplies. This is because it can kill bacteria and micro-organisms so thewater is safe to drink. Itis also used in the manufacture of bleach and PVC. Bauxite Bauxite is the main ore for aluminium. The bauxite is melted down to formthe electrolyte needed for electrolysis. When the currentis applied, the molten aluminium will sink to the bottom. Test for chlorine Chlorine bleaches damp litmus paper white Test for hydrogen When a lighted splint is applied, it will make a squeaky pop sound if hydrogen is present. Test for oxygen A glowing split will relight in a test tube of oxygen.
- 5. Extraction from ore Where a metal is on the reactivity series effects how it is extracted. If it is below carbon, it can be extractred by a reduction reaction with carbon. This is done by heating the metal with carbon. Metals more reactive than carbon are extracted using eloectrolysis. Metals such as gold are found unombined. This meand it can be easily removed fromits ore. Oxidisation and reduction In the oxidation and reduction reaction below. Copper dioxide is reduced. This means oxygen is removed. Carbon is therefore oxidised. This means it is gaining oxygen. Properties of Metals Typical properties: Strong (but can be bent or hammered into shape) Conduct heat Conduct electricity Aluminium: Low density Corrosion-resistant Purealuminium isn’tstrong but can be in alloys
- 6. Copper: Hard Strong High melting point Good conductor of electricity Good for water pipes (Below hydrogen in the reactivity series so doesn’t react with water) Gold: Shiny Easy to shape Non-reactive Corrosion of Metals Metals higher up in the reactivity series are more likely to corrodeas they react more easily with oxygen Iron Iron that is extracted in a blast furnaceis only 96% iron. These impurities make the iron brittle so it doesn’t havemany uses. When these impurities are removed, the iron atoms are all the same size. The layers of iron atoms can then slide over each other. This means the iron is too bendy for most uses. Steel Steel is produced when small amounts of carbon are added to iron to form an alloy. Shape memory alloys These are metal alloys that can go back to its original shape. An example of this is nitinol. This is made fromnickel and titanium. When heated, it returns back to its original shape. This can be used in glasses frames or stents for damaged blood vessels.
- 7. Hydrocarbons A hydrocarbon is an atom made up of only hydrogen and carbon. Itcan be found in crudeoil. Hydrocarbons can beseperated int sizes using fractional distilation Fractional Distilation Fractional Distilation Uses Complete Combustion Occurs when there is enough oxygen
- 8. Incomplete Combustion Occurs when there is not enough oxygen Choosing Fuels When choosing a fuel there are 4 things you need to consider: Ease of ignition Energy value (amount of energy released) How much ash and smokeit leaves behind Storageand transport Problems of Burning Fossil Fuels When fossilfuels are burnt, carbon dioxide and water vapour are released into the air. Sulfur impurities are also released into the atmosphere. If there is not enough oxygen, Itwill not burn properly and will release carbon sootand carbon monoxide into the atmosphere. Acid Rain Acid rain is formed when sulfuric acid from power stations and cars etc. mix with clouds to form dilute sulfuric acid. This falls as acid rain. Acid rain can acidify lakes and ponds killing plants and animals that live in it. Itcan also damage trees, limestone buildings and stone statues. Sulfur can be removed from fuels before they are burnt. This however, costs more money and uses more fossilfuels to do so. Acid gas scrubbers usecalcium carbonateto clean up fumes frompower stations before it enters the environment. Cars are now fitted with catalytic converters turn harmful gases into cleaner gases. Greenhouse Gases Greenhousegases such as carbon dioxide, methane and water vapour, actas an insulating layer that blocks in radiation fromthe sun. This is causing our planet to heat up (global warming).
- 9. Reducing Carbon Dioxide Levels Iron seeding Iron is needed for photosynthesis. If itis injected into the upper levels of the sea, then the growth of phytoplankton is promoted. These phytoplankton remove Carbon dioxide fromthe air through photosynthesis and put out oxygen. However, somephytoplankton grow to become toxic. Micro-organisms that feed on dead plankton useup oxygen. This creates ‘dead zones’ in the sea wherenothing can live. Converting Carbon Dioxide to Hydrocarbons Scientists have been trying to find a way to convertcarbon dioxide back into hydrocarbons. They havemanaged to convertcarbon dioxide into small chains of hydrocarbons butmaking long chains needed for petrol is more challenging. Biofuels Biogas Biogas is produced when micro-organisms decomposeanimalwasteor dead plants. This is becausethey build up carbon dioxide when they are alive, and when they die, this is released. This biogas can be burnt to produceheat to turn a turbine or can be used for heating Advantages Disadvantage Renewable Less land to grow food Easy to replace as crops grow quickly Large storagespace Carbon neutral (No carbon is released into the atmosphere) Highly flammable (hard to store) Clean fuel (there are few pollutants released) Cheap
- 10. Bioethanol Sugar beet and sugar canecan be fermented with yeastto form ethanol (alcohol). If you add 90% petrol to it, you create Gasohol. Cars can run from this. Advantages Disadvantage Less crude oil is required Less land to grow food Carbon neutral (Doesn’trelease carbon dioxide) Requires suitable climate to grow the sugar beet and sugar cane Clean fuel (Few pollutants) Distillation is needed after fermentation. This uses electricity Pollutants include carbon dioxide and water vapour. Theseare greenhouse gases. Hydrogen fuel cell Like a battery, it can produce energy. Ittakes in hydrogen and oxygen and creates water and energy. Advantage Disadvantage No pollutants Gases require large storage space No recharging required Flammable (Hard to store) More than 80% efficient Hydrogen still needs to be obtained through electrolysis. Less energy lost through heat and friction Measuring energy content in fuels 1. Measurefuel mass before 2. Measureout volume of water into a beaker 3. Add a draftshield to preventthe flame frombeing blown away fromthe water 4. Measuretemperature of the water 5. Light the fuel until the water rises 10 degrees 6. Then blow out the flame and re-measurethe fuel. 7. Calculate the difference 8. Repeat with another fuel The fuel that has used the least fuel is the bestfuel.
- 11. Cracking Heat and a catalyst are applied to long chains of hydrocarbons. This causes them to break into smaller chains. This process is usefulas long chains of hydrocarbons havelimited uses. 1. Heat is applied to the alkane and the catalyst 2. As the alkane passes over the catalyst, it will crack into smaller hydrocarbons. 3. These smaller hydrocarbons go down the delivery tube and into the jar. When you heat the boiling tube, it expands. If you kill the heat, the glass will shrink back to its original size. This creates a vacuumwhich sucks in the cold water. When the cold water comes into contact with the hot glass, the glass will shatter. Polymerisation Polymers aremade up of repeated links called monomers. Pressureand a catalyst are applied to alkenes. This breaks open the double bond, allowing it to join other monomers. When ethene is turned into a polymer, poly (ethene) is produced. Chemical Formula Monomers Polymers
- 12. Uses of synthetic polymers Problems with plastics Solutions
- 13. Alkanes and Alkenes Alkanes are saturated and doesn’tchange the colour of bromine water. Alkenes areunsaturated and decolourises bromine water. Drawing alkanes 1. Draw the carbons 2. Draw the bonds. Alkanes haveone double bond. 3. Carbons can only have 4 bonds. Count how many they have already used up and add more so it has a total of 4 bonds
- 14. 4. Then add the Hydrogens Cracking Thermal decomposition reaction. This can break down long chains of hydrocarbons into smaller more usefulhydrocarbons. This can be done by the useof heat and a catalyst. 1. Heat the alkane and catalystat the same time. 2. As the alkane starts to condense, it will pass over the catalyst. This causes it to crack into alkenes. 3. The alkenes will then pass the through the delivery tube and up into the beaker. 4. The water will be pushed out of the beaker and you will be left with alkenes. 5. You can then test it with bromine water. Itshould decolourise. This proves that it is an alkene not an alkane. Polymerisation Polymers arelong chains of repeated monomers. Thesecan be created by the use of pressureand a catalyst. An example of this is the monomer glucose forming the polymer starch.
- 15. The polymer, polyethene can be created when the double bond in the monomer, ethene breaks open and allows many other monomers to join. Polymer properties anduses toremember-