Molecular orbital theory.pptx
•Transferir como PPTX, PDF•
0 gostou•29 visualizações
CHAPTER: Chemical bonding 11 standard chemistry CBSE,ICSE ,state board. The ppt covers the whole concept of molecular orbital theory.
Denunciar
Compartilhar
Denunciar
Compartilhar
Recomendados
God is a creative God Gen 1:1. All that He created was “good”, could also be translated “beautiful”. God created man in His own image Gen 1:27. Maths helps us discover the beauty that God has created in His world and, in turn, create beautiful designs to serve and enrich the lives of others.
Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...

Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...christianmathematics
Mais conteúdo relacionado
Semelhante a Molecular orbital theory.pptx
Semelhante a Molecular orbital theory.pptx (20)
Último
God is a creative God Gen 1:1. All that He created was “good”, could also be translated “beautiful”. God created man in His own image Gen 1:27. Maths helps us discover the beauty that God has created in His world and, in turn, create beautiful designs to serve and enrich the lives of others.
Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...

Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...christianmathematics
Último (20)
Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...

Explore beautiful and ugly buildings. Mathematics helps us create beautiful d...
Measures of Central Tendency: Mean, Median and Mode

Measures of Central Tendency: Mean, Median and Mode
Disha NEET Physics Guide for classes 11 and 12.pdf

Disha NEET Physics Guide for classes 11 and 12.pdf
Call Girls in Dwarka Mor Delhi Contact Us 9654467111

Call Girls in Dwarka Mor Delhi Contact Us 9654467111
Ecosystem Interactions Class Discussion Presentation in Blue Green Lined Styl...

Ecosystem Interactions Class Discussion Presentation in Blue Green Lined Styl...
9548086042 for call girls in Indira Nagar with room service

9548086042 for call girls in Indira Nagar with room service
Kisan Call Centre - To harness potential of ICT in Agriculture by answer farm...

Kisan Call Centre - To harness potential of ICT in Agriculture by answer farm...
Molecular orbital theory.pptx
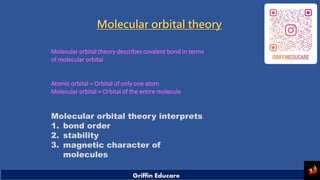
- 1. Molecular orbital theory Molecular orbital theory describes covalent bond in terms of molecular orbital Atomic orbital = Orbital of only one atom Molecular orbital = Orbital of the entire molecule Molecular orbital theory interprets 1. bond order 2. stability 3. magnetic character of molecules
- 2. MO theory considers the wave nature of electron electrons have a dual nature particles and waves Waves interact in two ways Constructive interaction Destructive interaction
- 3. Therefore electron interacts in 2 ways Constructive way Bonding molecular orbital lower energy most stable Destructive way Antibonding molecule orbital higher energy less stable Bonding molecular orbital is of lower energy than individual atomic orbital that’s why bond formation is exothermic
- 4. P orbital
- 5. PI BOND FORMATION SIGMA BOND FORMATION
- 7. How are molecular orbitals filled? Orbital’s are filled in order of increasing energy
- 10. Magnetic characteristics Paired electrons unpaired electrons Diamagnetic paramagnetic
- 11. MO DIAGRAM OF OXYGEN MOLECULE
- 13. HOMO : Highest occupied molecular orbital LUMO : Lowest unoccupied molecular orbital
- 14. Bond Order The calculation that predicts the stability of the molecule 3 : TRIPLE BOND 2: DOUBLE BOND 1: SINGLE BOND 0 : NO BOND
- 16. Question Draw molecular orbital diagram of F2 molecule and answer the following questions 1. what is the bond order of F2 molecule? 2. is it paramagnetic ? 3.Write molecular orbital electronic configuration of F2 molecule?